Abstract
Clonal hematopoiesis of indeterminate potential (CHIP) arises from acquired mutations in hematopoietic stem cells, most frequently DNMT3A and TET2. Surprisingly, CHIP is associated with not only hematologic malignancy but also cardiovascular disease and an expanding array of inflammatory conditions with differential pathogenicity due to DNMT3A and TET2 mutations. In mouse models, CHIP increases risk of atherosclerosis in part due to signaling through the IL-6/IL-1β signaling axis. Here, we define the differential signaling response to IL-6 cytokine stimulation in human monocytes from patients with TET2 and DNMT3A CHIP.
We stimulated peripheral blood mononuclear cells ex vivo and performed single-cell RNA sequencing (scRNA-seq) on ~150,000 single cells. At basal levels, we observed that genes involved in inflammation, monocyte activation, cell adhesion, and antigen presentation were enriched in monocytes from patients with TET2 CHIP (n=4) compared to control patients without CHIP (n=3). Following IL-6 stimulation, we found inflammatory genes significantly upregulated in TET2 patient monocytes but not in DNMT3A patient monocytes (n=3 patients).
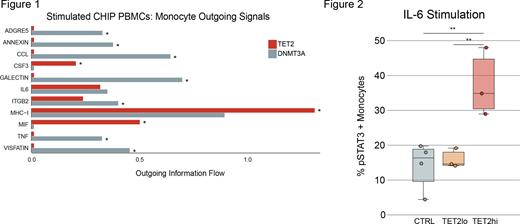
We used CellChat to further characterize cell-cell interactions in the IL-6 stimulated CHIP samples. We found that inflammatory ligands such as IL1B, CXCL3, and IL-8 were uniquely enriched in TET2-mutated monocytes after stimulation. We also identified inferred Macrophage Migration Inhibitory Factor (MIF) ligand signaling from TET2 monocytes was significantly higher than DNMT3A monocytes (Figure 1).
Larger CHIP clones confer increased disease risk. To test the hypothesis that IL-6 responses are elevated in high TET2 VAF samples, we performed phospho-specific flow cytometry on TET2 mutant samples at baseline and following IL-6 stimulation. We measured pSTAT3 positivity in high VAF samples (TET2hi, >25% VAF, n=3), low VAF samples (TET2lo, <25% VAF, n=3) and controls (n=4). Despite all samples exhibiting response to stimulation, TET2hi monocytes displayed the highest proportion of pSTAT3+, significantly higher than both TET2lo and control samples (Figure 2, ANOVA p= 0.008, Tukey HSD p=0.008 (CTL vs. TET2hi) and p=0.02 (TET2lo vs. TET2hi)).
Combining scRNA-seq with genotyping presents the opportunity to compare CHIP mutated and non-mutated cells from within a single patient. To assign clonality within the monocyte population, we performed MAESTER, a mitochondrial lineage tracing technique, in parallel with nuclear DNA genotyping. We then sought to isolate active transcription factor networks using pySCENIC, a tool that derives active transcription factors and their downstream targets from scRNA-seq data. For our analysis, we selected a TET2 mutant sample with high VAF (51%). In the stimulated monocyte condition, we discovered 237 active regulons within the mutant population. Notably, the XBP1 regulon, a known regulator of MHC-II expression, had the highest regulon specificity score (RSS), indicating activation in TET2-mutated cells. The SPI1 regulon, involved in myeloid lineage determination, the pro-inflammatory regulon, REL, and hypoxia-inducible factor HIF1A also had high RSS scores.
We demonstrate that TET2 mutant monocytes exhibit unique signaling responses in response to IL-6 stimulation compared to both control samples and non-mutated cells from within the same patient. High VAF TET2 mutant samples displayed an elevated pSTAT3 response compared to lower VAF and control samples, suggesting that IL-6 signaling is a constitutively active, cell-intrinsic property. Our phospho-specific flow cytometry method has potential diagnostic and prognostic utility in stratifying individual risk for inflammation-related consequences of CHIP, such as cardiovascular disease and malignant transformation. Future prospective randomized clinical trials are required to test whether CHIP patients with TET2 mutation status and high VAF would benefit from IL-6/IL-1β interventions.
Disclosures
Van Galen:ManaT Bio: Consultancy; Immunitas: Honoraria. Savona:Novartis: Consultancy; Astex Pharmaceuticals: Research Funding; Forma: Consultancy; TG Therapeutics: Consultancy, Other: Travel expenses, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Other: travel expenses; Incyte Corporation: Research Funding; Taiho Pharmaceutical: Consultancy; Karyopharm Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; Takeda: Consultancy; ALX Oncology: Research Funding; Sierra Oncology: Consultancy, Other: travel expenses. Bick:TenSixteen Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Ferrell:Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal